Atoms & Molecules
Atoms: smallest stable units of matter
Protons = + charge, are designated p+
Electrons = - charge, are designated e–
Neutrons = no charge, are neutral.
Atomic mass refers to the number of protons & neutrons
Atomic number: number of protons in the nucleus of the atom
Isotopes: one of the different forms of an element, distinguished from one another by different numbers of neutrons.
Common Essential Elements
Oxygen ~65% of total body weight: Is also abundant, needs 2 electrons to fill outer shell (valence)
Carbon < 20%: Is abundant, it tends to maximally share electrons. Likely basis for any life in-universe. Can form complex structures.
Hydrogen < 10%
Nitrogen <3%
Calcium < 2%
Potassium, Sodium, Chloride, Magnesium, Sulphur, Iron, and Iodine total < 1%
Trace elements include Fluorine, Manganese, Zinc, Selenium, Aluminum, Boron, Cadmium, Copper, and a few others
Chemical Bonds
3 main types of bonds b/w atoms:
① Ionic
② Covalent
③ Hydrogen bond
1. Ionic bonds
Ionic bonds form when an atom loses or gains a valence electron. Ions are formed.
Positively & negatively charged ions are attracted to one another.
Cations are positively charged ions that have given up one or more electrons (they are electron donors.)
Anions are negatively charged ions that have picked up one or more electrons than another atom has lost (they are electron acceptors).
2. Covalent bonds
Covalent bonds are formed by atoms of molecules sharing 1, 2, or 3 pairs of their valence electrons.
Covalent bonds are the strongest chemical bonds. Single, double, or triple covalent bonds are formed by sharing one, two, or three pairs of electrons, respectively.
Covalent bonds may be nonpolar or polar
In a nonpolar covalent bond, atoms share the electrons equally; nonpolar covalent bonds are the most common types of covalent bonds, as shown in this graphic depicting hydrogen gas, oxygen gas, nitrogen gas, and methane gas.
Polar covalent bonds are formed by the unequal sharing of electrons between atoms.
Polar covalent bonds are extremely important because the all-important water molecule makes use of this bond.
In water, oxygen attracts the hydrogen electrons more strongly, making oxygen slightly electronegative as indicated by the negative Greek delta sign
3. Hydrogen bonds
Hydrogen bonds are weak interactions (approximately 5% as strong as covalent bonds) between hydrogen & adjacent electronegative atoms like oxygen or sulfur.
Hydrogen bonds result from the attraction of oppositely charged parts of molecules—they should not be confused with covalent bonding to hydrogen which involves the actual sharing of electrons.
*water also repel molecules with nonpolar covalent bonds, like fats, lipids, & oils.
Hydrogen bonds are useful in establishing links between molecules or between distant parts of a very large molecule. Large 3-D molecules (like proteins) are often held together by a great many hydrogen bonds
In water, hydrogen bonding provides considerable cohesion which creates a very high surface tension (as this bug demonstrates)
*Characteristics of water
• Cohesive – water flows
• High heat capacity- i.e. can absorb (lots of energy before hydrogen bond breaks)
• Interact with an ionic compound
Inorganic & organic compounds
Inorganic: either no carbon or 1 carbon in molecule / does not contain both carbon & hydrogen but contain only carbon or only hydrogen
e.g. water, salt, acid (H2O, NaCl, HCL, CO2)
Organic: contains 2 or more carbons linked together or both carbon & hydrogen
Inorganic compounds
Inorganic compounds are structurally simple molecules that usually lack carbon – like the salt potassium chloride (KCL) depicted here
Organic compounds
Always contain carbon & usually large, complex, molecules. Usually contain hydrogen
Always have covalent bonds
Organic compounds are synthesized via covalent bonds within living organisms, including the human body. It typically consists of groups of carbon atoms covalently bonded to hydrogen, usually oxygen, and often other elements as well.
Biochemistry (chemistry of the reaction of life)
Carbohydrates
Lipids
Proteins
Nucleic Acids
1. Carbohydrates
Carbohydrate is a molecule composed of carbon, hydrogen, & oxygen
Most common are 5 carbons or 6 carbon molecules (sugar), most common is glucose C6H12O6
Carbohydrates provide most of the energy needed for life and include sugars, starches, glycogen, & cellulose.
Some carbohydrates are converted to other substances that are used to build structures and to generate ATP.
Other carbohydrates function as food reserves.
Carbohydrates are divided into three major groups based on their size: monosaccharides, disaccharides, and polysaccharides.
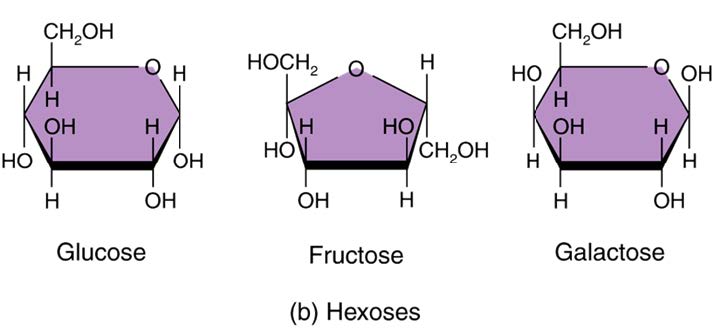
Monosaccharides (Carbohydrates)
− Monosaccharides are the simplest sugars:
− 5 carbon - Carbon sugars are used in nucleic acids / 6 carbon sugars are the most easily recognizable in our diet.
Glucose: molecule primarily used in cellular respiration to generate energy (ATP)
Galactose
Fructose
Disaccharides
− Disaccharides are made by combining 2 monosaccharides by removing a water molecule (dehydration synthesis)
Sucrose (table sugar) = glucose + fructose
Lactose (milk sugar) = glucose + galactose
Maltose = glucose + glucose (grain)

Polysaccharides
− Polysaccharides are the largest carbohydrates and may contain hundreds of monosaccharides.

Glycogen: polymer of glucose
Stored in the tissue of animals – muscle & liver
*glucose dissolves in water very easily but glycogen is not very water-soluble so, the human body can store it more easily.

Cellulose: unlike starch,
Cellulose – have no branch but *parallel bundle. – No animal can digest it – human can’t digest it but we have it for fibers (fluffy stool), some animal has bacteria in their gut

Starch: polymer of glucose
Relatively easy to digest, similar to glycogen- how plants store glucose.
2. Lipids
Lipids are another major group of organic molecules.
Like carbohydrates, they contain carbon, hydrogen, and oxygen;
unlike carbohydrates, they do not have a 2:1 ratio of hydrogen to oxygen.
They have few polar covalent bonds, which makes them hydrophobic and mostly insoluble in water.
They combine with proteins (lipoproteins) for transport in the blood.

Triglycerides (glycerol+3 fatty acid)
− Human body stores lipids as triglycerides - store it as adipose tissue (fat tissue) and provide protection, insulation, and energy (both immediate and stored).
− At room temperature, triglycerides may be either solid (fats) or liquid (oils).
− Triglyceride storage is virtually unlimited.
− Excess dietary carbohydrates, proteins, fats, and oils are deposited in adipose tissue as triglycerides.
− Triglycerides provide more than twice as much energy per gram as either carbohydrates or proteins.
Phospholipids
− Phospholipids are important membrane components.
− Both polar and nonpolar regions make them soluble in both water & fats ( amphipathic— both hydrophilic & lipophilic.)

− They have a polar head formed from a phosphate group (PO4-3) & a glycerol molecule (forms H-bonds with water), & 2 nonpolar fatty acid tails that interact only with lipids. 2 fatty acids chains + phosphate +glycerol
− Phospholipids have a polar head and 2 non-polar tails:
− This forms biological membrane – phospholipids bilayers- it forms a boundary around cells- allows for an internal environment that is very different from the external environment
Steroids

− Steroids are lipids molecules that have four rings of carbon atoms.They include:
→ Sex hormones
→ Bile salts
→ Some vitamins
→ Cholesterol, which serves as an important component of cell membranes & as starting material for synthesizing other steroids Steroids are based on the lipid cholesterol molecule. They include the molecules used as sex hormones, as well as other hormones used in coping with stress (cortisol).
3. Proteins
Proteins are complex macromolecules that are polymers of many subunits called amino acid (that contain carbon, hydrogen, oxygen, & nitrogen.)
The covalent bond linking 2 amino acids together is called a peptide bone
The assembled polymer is called a poly peptide

They are the most “human” of all organic compounds. We can rightly say that humans are protein creatures, using carbohydrates to burn as fuels, & lipids for structural support, energy storage, & hormones.
This graphic is a model of an enzyme protein.
Proteins are constructed from combinations of different amino acids.
Amino acids are small molecules with a simple basic structure: a carbon atom to which following 3 groups are added

− An amino group (-NH2)
− A Carboxyl group (-COOH)
− A functional group (R)
20 different types of amino acid – differ based on R group (All amino acids (A.A) have the same basic structure— only the “R” group changes.)
**Function of proteins
− Energy source
− Structural molecule
− Enzymes
− Transport molecule
− Osmolarity
Dipeptides
− Dipeptides are formed from 2 amino acids joined by a covalent bond called a peptide bond.
− This process involves dehydration synthesis.
Polypeptide
− Polypeptide chains contain 10 to 2000 amino acids.
4 levels at which proteins are structurally organized:

(1) Primary (10)
(2) Secondary (20)
(3) Tertiary (30)
(4) Quaternary (40)
The resulting shape of the protein greatly influences its ability to recognize & bind to other molecules.
Denaturation (loss of protein structure) by a hostile environment causes loss of its characteristic shape and function. (denatured protein is inactive)
*Increased temperature or lower pH affects hydrogen bonding as well as hydrophobic interactions, which are involved in the folding process.

E.g. An egg white turning solid white when exposed to high temperatures is an example of protein denaturation.
Enzymes are special proteins that catalyze (speed up) metabolic reaction in all living cells.

− Regulate when reaction will happen
− Increase rate of reaction
− Allows to reaction to occur at normal temperature
− The substrate is the substance upon which an enzyme has its effect. In this regard, enzymes are highly specific. (lock & key mechanism)
4. Nucleic Acids
the basic units of nucleic acids
Nucleotide is made of
− 5 Carbon sugar – pentose sugar (deoxyribose sugar or ribose)
− Phosphate group
− Nitrogen containing base:
Adenine, guanine – purine
Thymine (or Uracil-substitute in RNA), cytosine – pyrimidine
Nucleotides is
− Linked into chains by binding sugar to sugar
− 2 chains interact form double chain, chain twists – form a helix shape – double helix− Two chains held together by interactions between bases.
-> Adenine-Thymine (Uracil)
-> Guanine-Cytosine
− Sequences of nucleotides contain info (code) to spell out how to build protein from amino acids
− Need enzyme to separate parts of double helix to make smaller copies of parts of DNA
Transcription (DNA is converted into RNA code) → Translation (RNA to protein)
Nucleic Acids

Nucleic acids are long chainlike molecules composed of a series of nearly identical building blocks called nucleotides
These molecules carry genetic information as deoxyribonucleic acid (DNA) & ribonucleic acid (RNA).
DNA molecules remain inside the nucleus of cells and are the “master” template of our genetic code.
RNA is a slightly different nucleic acid macromolecule that relays instructions from the nucleus to guide assembly of amino acids into proteins in the cytoplasm.
ATP (Adenosine Tri-Phosphate)

Structure: P-P-P-Adenine
Nucleic acids are used in making energy-carrying molecule in the body called adenosine triphosphate (ATP).
Supplies each action of breaking each bond to just the right/appropriate amount of energy.
If need energy break phosphate bone ATP→ADP→AMP.
− Synthesis of ATP is catalyzed by the ATP synthase enzyme which adds the terminal high energy phosphate bond (often depicted as ~P as opposed to a regular –P bond) to ADP.
→ Energy from 1 glucose molecule is used during both anaerobic & aerobic respiration to create 36 to 38 molecules of ATP.
'간호학과 공부자료 > Anatomy & Physiology' 카테고리의 다른 글
| 1. An introductory to Human Body (0) | 2020.10.25 |
|---|

댓글